Anis
Currently pursuing Ph.D. at the University of South Carolina, Columbia, SC.
Research Focus:
- Synthesis of 1,3,4-Oxadithiazole
- Synthesis of Fluoro-substituted Schiff Bases from 2,9-dimethyl-1,10-phenanthroline dialdehyde and sulfur-containing amines

Oxadiazoles and their derivatives are important classes of heterocyclic compounds and have created greater interest in synthetic organic and medicinal chemistry due to their versatile medicinal potential1. 1,3,4-oxadiazoles ring containing compounds display a remarkable broad spectrum of biological activity such as virucidal2, CNS depressant3, genotoxic4, anticonvulsant5, insecticidal6, antitubercular7, anti-HIV8, herbicidal9, anti-inflammatory10 and anticancer11-16 activities. They have also found to exhibit antimalarial17, muscle relaxants18, antitumour19, lipid peroxidation inhibitor20, tyrosinase inhibition21, antimicrobial22,23, and remarkable analgesic24,25, anti-convulsant26, diuretic, hypnotic and sedative properties27. Therefore, 1,3,4-oxadiazoles have attracted the researchers all over the world to synthesize these classes of compounds by employing traditional methods, introducing new innovative methods and techniques and study their biological applications.
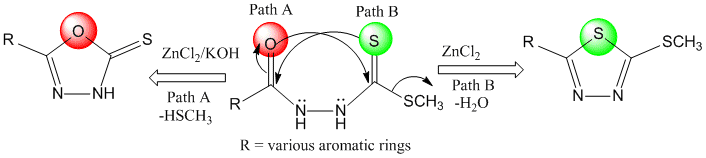
1,3,4-oxadiazole-2-thione are traditionally synthesized by base catalyzed cyclization reactions of corresponding amides. The conventional method of synthesizing 1,3,4-oxadiazole-2-thione from acid hydrazide using KOH in ethanol and CS2 28 or pyridine and CS2 29. The most common procedure for the synthesis of 1,3,4-oxadiazole-2-thione is base (Et3N, EtOH) catalyzed intramolecular cyclization from acyl-dithiocarbazates30. 1,3,4-oxadiazole-2-thiols (Which are tautomers of corresponding thiones)31 were synthesized by using CS2 in presence of N-methyl morpholine in ethanol32. Earlier people synthesized 1,3,4-oxadiazole-2-thione attached with nitrogen containing five and six member rings and also quinoline16. Besides this, most of the synthetic methods are involved forcing reaction conditions like high temperature, time consuming, and purification problems.
Publications:
1] Synthesis of Loratadine and some of it's amide derivatives as cytotoxic agents. Lipiar Khan Mohammad Osman Goni, Nahida Akter, Md. Rabiul Islam, Anisur Rahman, Mohammad Karim. International Journal of Medicinal Chemistry & Analysis, 2015, vol 5, issue 2, pp 68-73.
2] Zn(II) Catalyzed Efficient Synthesis of 1,3,4-Oxadiazole and 1,3,4-Thiadiazole. Md. A. Rahman, Mohammad R. Karim**, Md. Arifuzzaman, Tasneem A. Siddiquee*, Aminul H. Mirza. Tetrahedron Letters, 2014, 55, 3267-73.
3] 2,9-Bis(5-sulfanylidene-4,5-dihydro-1,3,5-oxadiazol-2-yl)-1,10phenanthroline dimethyl sulfoxide disolvate. Md. A. Rahman, Mohammad Karim*, Md. Arifuzzaman, Tasneem Siddiquee and Lee M. Daniels. Acta Cryst. (2014), E70, 0321-0322.
webpage contact:
Chemistry


